Virta's impact on NAFLD, the $100B problem affecting 60% of people with T2D

While our mission at Virta is to reverse type 2 diabetes in 100 million people by 2025, we’ve also shown promising results in related chronic diseases for people living with type 2 diabetes. Our latest peer-reviewed results continue this trend. Today, BMJOpen published changes in surrogate markers of non-alcoholic fatty liver disease (NAFLD) at 1 year in patients with type 2 diabetes (T2D) from Virta’s ongoing clinical trial. The results demonstrate the promise of the Virta Treatment as one of the most effective therapies for NAFLD for people living with T2D.
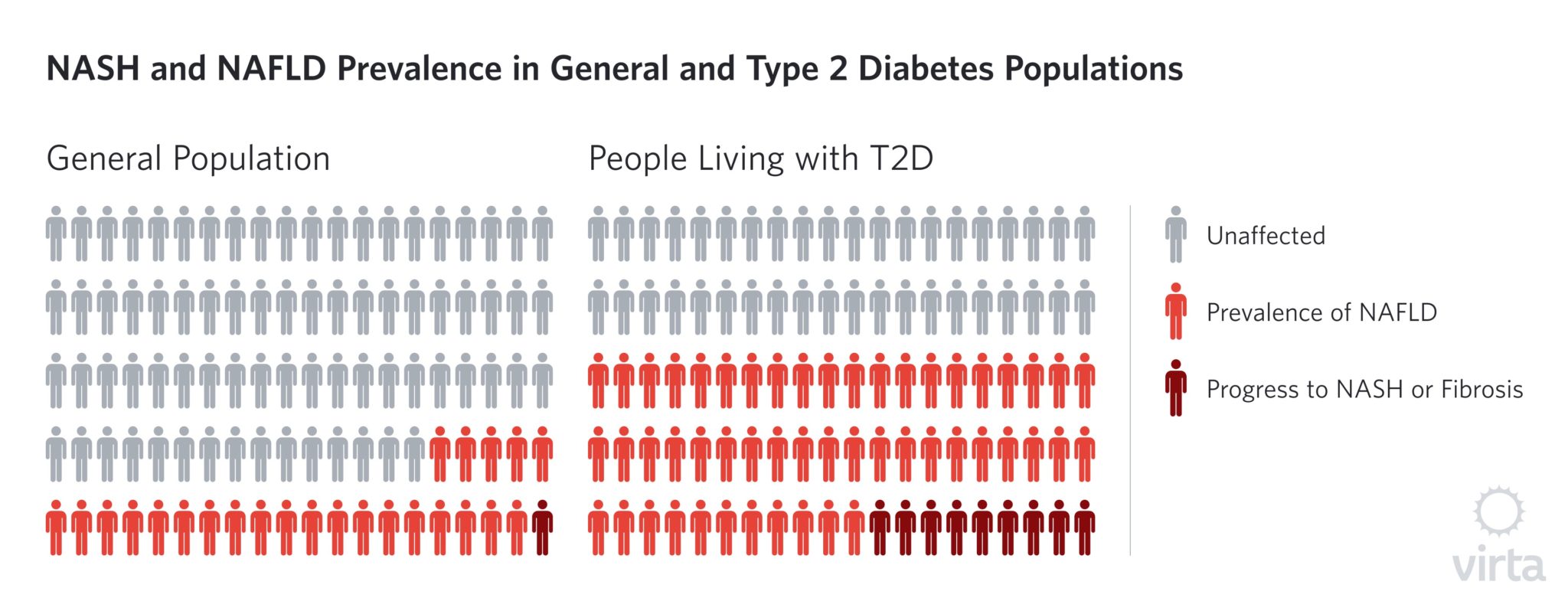
Why is this significant? NAFLD is a particularly devastating disease, costing the U.S. an estimated $103 billion per year. Nearly 60% of people living with type 2 diabetes also have NAFLD. Despite these alarming statistics, there are currently no approved pharmaceuticals to treat the the disease.
Furthermore, most people living with NAFLD do not know they have it: NAFLD is often asymptomatic until it has progressed to cirrhosis or liver failure. In addition to patients, clinician awareness of NAFLD as a disease is also low. NAFLD as a term is less than 20 years old, and diagnostic criteria only started to be developed in 2005.
Here are 3 reasons I am excited about the Virta Treatment’s effect on NAFLD:
NAFLD is a common comorbidity of T2D
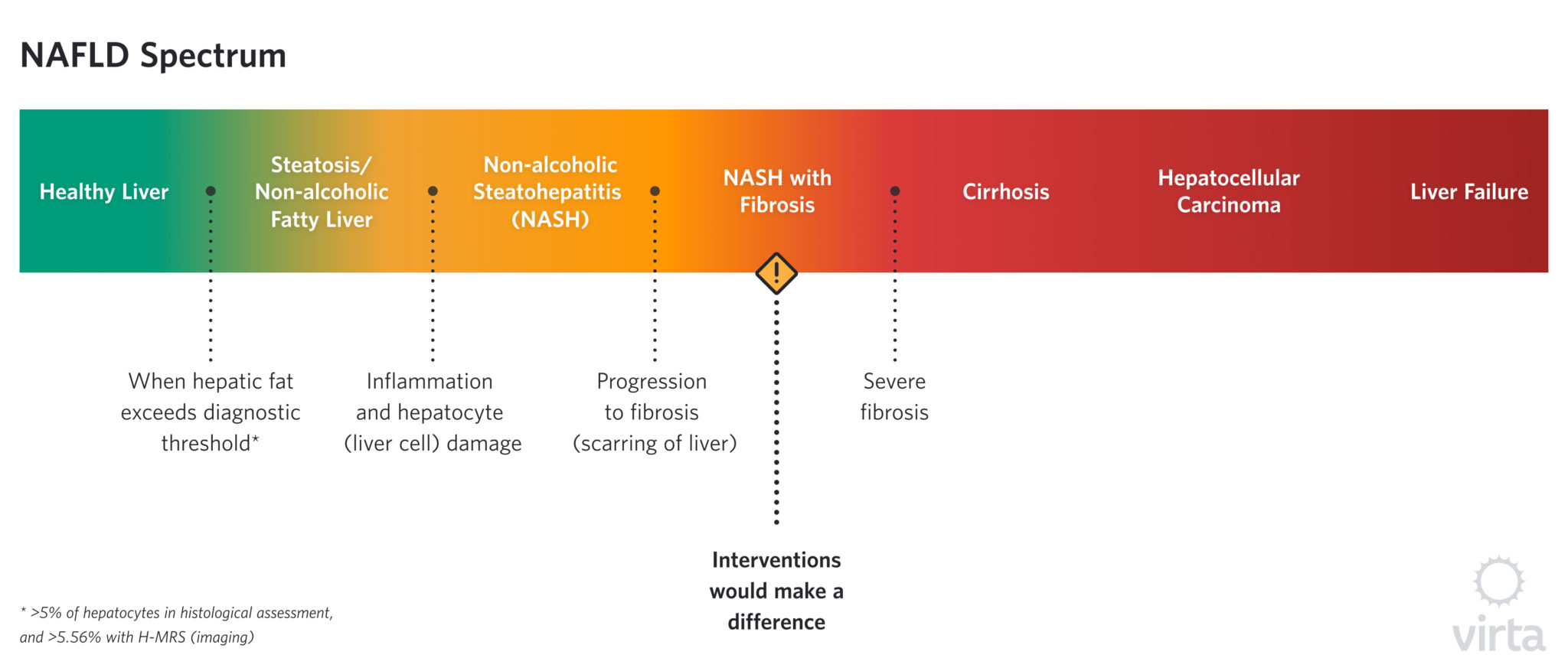
NAFLD describes a spectrum of disease states that vary in severity of liver fat content (steatosis), inflammation, and scarring (fibrosis). Over time, the damage from NAFLD may progress to cirrhosis or even liver failure, the latter of which requires a liver transplant for survival.
NAFLD is particularly concerning for people living with T2D because it is directly correlated with poor glycemic control, and the prevalence rate of NAFLD is significantly higher in people living with T2D compared to the general population.
Additionally, an individual living with T2D and NAFLD as comorbidities has an increased susceptibility of developing more severe forms of NAFLD, such as NASH and liver fibrosis, as well as an increased likelihood of macro- and microvascular complications like cardiovascular disease and retinopathy.
In total, the direct medical cost of managing NAFLD and its associated complications are estimated to be over $100 billion per year in the United States, and they are projected to further increase.
There is only one treatment option for NAFLD
There are currently no approved pharmacological interventions for NAFLD or its more severe variant, NASH. And even though NAFLD is very common in people with type 2 diabetes, the current ADA guidelines do not require screening for NAFLD. The only approved treatment approach for NAFLD set forth by the AASLD and EASL (American Association for the Study of Liver Diseases and European Association for the Study of the Liver) is to lose weight through lifestyle intervention: >5% weight loss is associated with improvement in steatosis, and >7% is necessary for improvement of NASH. These degrees of weight loss have been associated with improvements in different stages of NAFLD, with greater weight loss effective in resolving inflammation, NASH, and regressing fibrosis.
The Virta Treatment exceeds NAFLD treatment guidelines
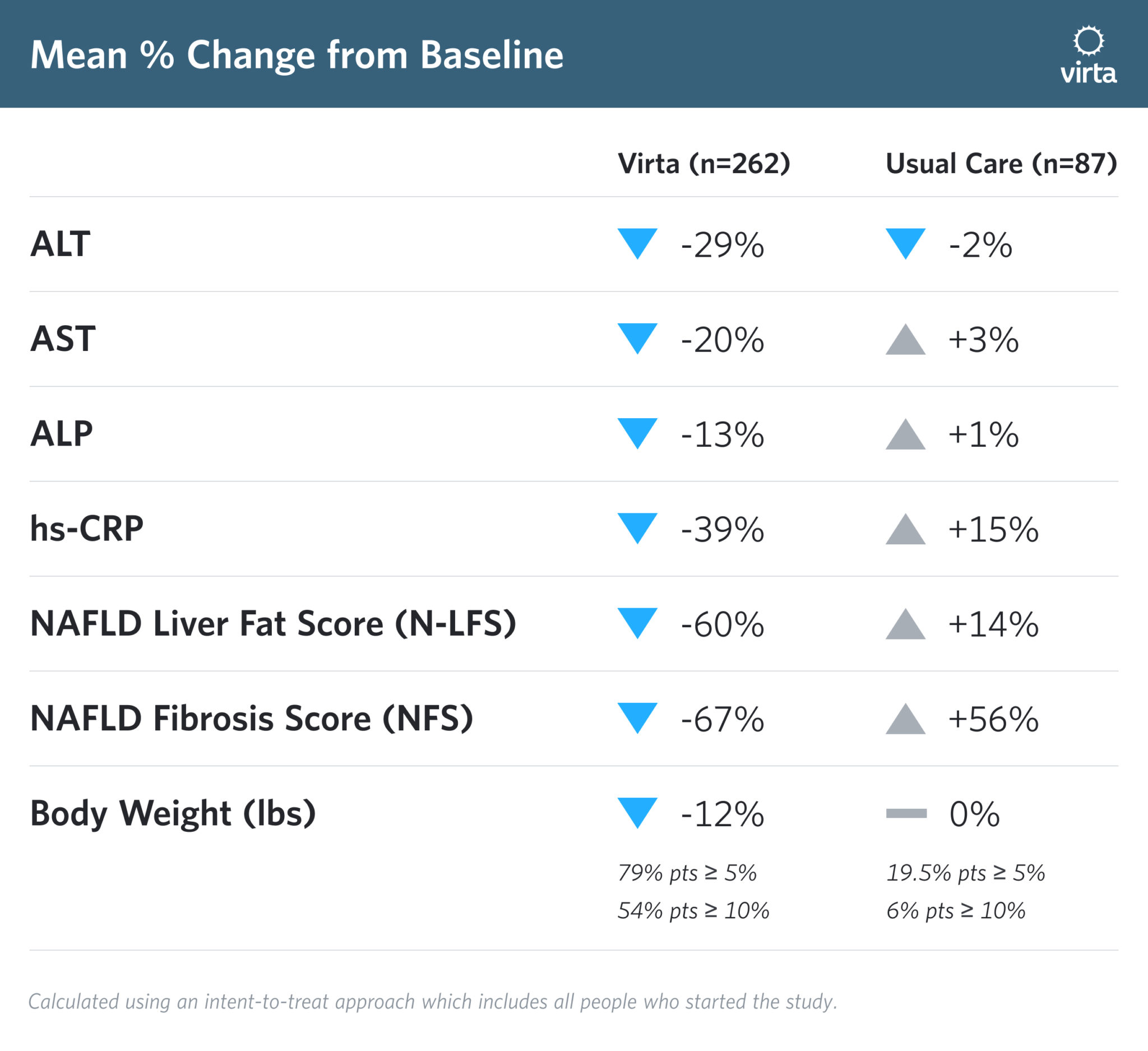
At 1 year, Virta’s clinical trial patients not only met, but exceeded, AASLD and EASL guidelines, and our trial patients experienced significant improvements in non-invasive scores for steatosis and fibrosis.
On average, our clinical trial patients were able to maintain a 12% loss of their starting weight at 1 year. That means more than half of Virta's trial patients lost more than the weight necessary for improvement of NASH. We used non-invasive scores to measure the Virta Treatment’s effect on steatosis and fibrosis with the NAFLD Liver Fat Score (N-LFS) and NAFLD Fibrosis Score (NFS), respectively.
We have already demonstrated that the Virta Treatment can reverse type 2 diabetes, while simultaneously improving inflammation, cardiovascular disease risk factors, and BMI. These latest results have us incredibly excited about the Virta Treatment’s promise for improving liver function, too, all while safely reducing, rather than adding, to a person’s prescriptions.
We will be publishing other results from our clinical trial and commercial populations soon. In the meantime, if you want to become or refer a patient to our treatment, please apply at https://www.virtahealth.com/.
This blog is intended for informational purposes only and is not meant to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition or any advice relating to your health. View full disclaimer
Are you living with type 2 diabetes, prediabetes, or unwanted weight?







.jpg)

